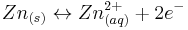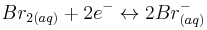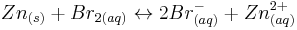Zinc–bromine battery
| specific energy | 34.4–54 W·h/kg (124–190 J/g) |
|---|---|
| energy density | 15.7–39 W·h/L (56.5–140 kJ/L) |
| Charge/discharge efficiency | 70%[1] |
| Energy/consumer-price | US$400/kW·h (US$0.11/kJ) |
| Cycle durability | >2,000 cycles |
| Nominal cell voltage | 1.8 V |
The zinc–bromine flow battery is a type of hybrid flow battery. A solution of zinc bromide is stored in two tanks. When the battery is charged or discharged the solutions (electrolytes) are pumped through a reactor stack and back into the tanks. One tank is used to store the electrolyte for the positive electrode reactions and the other for the negative. Zinc bromine batteries from different manufacturers have energy densities ranging from 34.4–54 W·h/kg .
The predominantly aqueous electrolyte is composed of zinc bromide salt dissolved in water. During charge, metallic zinc is plated from the electrolyte solution onto the negative electrode surfaces in the cell stacks. Bromide is converted to bromine at the positive electrode surface of the cell stack and is immediately stored as safe, chemically complexed organic phase in the electrolyte tank. Each fully recyclable high-density polyethylene (HDPE) cell stack has up to 60 bipolar, plastic electrodes between a pair of anode and cathode end blocks.
The zinc–bromine battery can be regarded as an electroplating machine. During charging zinc is electroplated onto conductive electrodes, while at the same time bromine is formed. On discharge the reverse process occurs, the metallic zinc plated on the negative electrodes dissolves in the electrolyte and is available to be plated again at the next charge cycle. It can be left fully discharged indefinitely without damage.
The primary features of the zinc bromine battery are:
- High energy density relative to lead–acid batteries
- 100% depth of discharge capability on a daily basis
- High cycle life of > 2,000 cycles at 100% depth of discharge, at which point the battery can be serviced to increase cycle life to over 3,500 cycles
- No shelf life limitations as zinc–bromine batteries are non-perishable, unlike lead–acid and lithium-ion batteries, for example.
- Scalable capacities from 10 kW·h (0.036 GJ) to over 500 kW·h (1.8 GJ) systems
- The ability to store energy from any electricity generating source
Three examples of zinc–bromine flow batteries are ZBB Energy Corporation's Zinc Energy Storage System (ZESS), RedFlow Limited's Zinc Bromine Module (ZBM), and Premium Power's Zinc-Flow Technology.
These battery systems have the potential to provide energy storage solutions at a lower overall cost than other energy storage systems such as lead-acid, vanadium redox, sodium–sulfur, lithium-ion and others.
Electrochemistry
At the negative electrode zinc is the electroactive species. Zinc has long been used as the negative electrode of primary cells. It is a widely available, relatively inexpensive metal which is electronegative, with a standard reduction potential, E° = −0.76 V vs SHE. However, it is rather stable in contact with neutral and alkaline aqueous solutions. For this reason it is used today in zinc–carbon and alkaline primaries.
In the zinc–bromine flow battery the negative electrode reaction is the reversible dissolution/ plating of zinc, according to the following equation.
At the positive electrode bromine is reversibly reduced to bromide, (with a standard reduction potential of +1.087 V vs SHE) according to the following equation.
The overall cell reaction is therefore.
The measured potential difference is around 1.67 V per cell (slightly less than that predicted from the standard reduction potentials).
The two electrode chambers of each cell are divided by a membrane (typically a microporous or ion-exchange variety). This helps to prevent bromine from reaching the positive electrode, where it would react with the zinc, causing the battery to self-discharge. To further reduce the self-discharge, and also to reduce the vapor pressure of bromine, complexing agents are added to the positive electrolyte. These react reversibly with the bromine to form an oily red liquid and reduce the Br2 concentration in the electrolyte.
References
- ^ "(Dead link) AN EVALUATION OF WINDFARM STABILIZATION AND LOAD SHIFTING USING THE ZINC-BROMINE BATTERY (ZBB)". Gridwise Engineering Company, Xantrex Technology, Inc., ZBB Energy Corporation. 12 December 2002. p. 10. http://www.zbbenergy.com/pdf/technicalpaper_evaluation.pdf. Retrieved 2009-07-27.
- RedFlow Limited
- ZnBr Batteries, Electricity Storage Association
Bromine Complexation in Zinc–Bromine Circulating Batteries D. J. Eustace, J. Electrochem. Soc. 127(3), 528–32 (1980)
Handbook of batteries, 3rd edition. D. Linden, T. B. Reddy. 39.1–39.8 (2002)